Familiarize staff with the regulatory benchmarks and tips governing Microbial Limit Testing. This ensures that testing procedures align with business prerequisites and retain compliance with applicable authorities.
The existence of numerous microbial in non-sterile products might trigger adverse functions, so microbial security is essential for medicine. Microbial security need to be deemed in the least stages of the event, manufacturing, and subsequent storage and use on the prescription drugs. The microbial limit test (MLT) can Examine the quantity of certain microorganisms are existing in non-sterile pharmaceutical samples from raw products to ultimate products, the test can decide if specified microorganisms are exceeded quantitative limits.
This growth brings about strains that are more difficult to regulate, complicating endeavours to ascertain productive microbial limits.
Step one in the Microbial Limit Test involves the watchful selection and collection of consultant samples. This method is crucial to obtaining accurate and meaningful final results.
Preserve specific information of all corrective steps taken. This documentation gives proof with the actions carried out to address the deviation and makes sure transparency in the course of regulatory inspections.
The one of a kind characteristic of fungi is their diverse metabolic pathways, which permit them to stop working complex substances. This adaptability can be a benefit in foods creation but might also complicate attempts to manage their expansion.
* Use deviations as possibilities for ongoing enhancement. Perform assessments and conversations to determine classes figured out from Each individual deviation, facilitating ongoing improvement of Microbial Limit Testing procedures.
Microbial limits Engage in a important check here job in numerous industries because they directly influence product or service security, buyer wellness, and regulatory compliance. In sectors including pharmaceuticals, cosmetics, and food creation, comprehension and taking care of microbial limits make sure products are safe to be used and free from harmful amounts of microorganisms.
Compliance with these frameworks makes sure that products and providers meet recognized safety expectations, allowing for international trade and purchaser protection.
This doc supplies an overview of capsule production in 3 sentences: It discusses the creation method at Bengal School of Engineering in microbial limit test definition India for any student named Ankush Biswas. It acknowledges the contributions of his supervisor and Many others who supported his undertaking on capsule production.
Vital milestones in microbial analysis have appreciably affected the institution of microbial limits. Notably, the invention of penicillin by Alexander Fleming in 1928 catalyzed a paradigm change inside the understanding of bacterial conduct and resistance.
Preservative Efficacy Testing: Every beauty merchandise ought to display adequate antimicrobial activity by way of demanding testing, frequently employing techniques like obstacle testing.
In summary, the responsibilities from the QC Office in Microbial Limit Testing are multi-faceted and integral to making sure the safety and high quality of Uncooked resources and finished products.
To circumvent contamination in the course of the sampling and testing method, the QC Office need to adhere to strict aseptic approaches.
 Barret Oliver Then & Now!
Barret Oliver Then & Now! Earvin Johnson III Then & Now!
Earvin Johnson III Then & Now!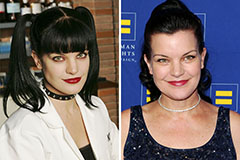 Pauley Perrette Then & Now!
Pauley Perrette Then & Now! Dolly Parton Then & Now!
Dolly Parton Then & Now! Peter Billingsley Then & Now!
Peter Billingsley Then & Now!